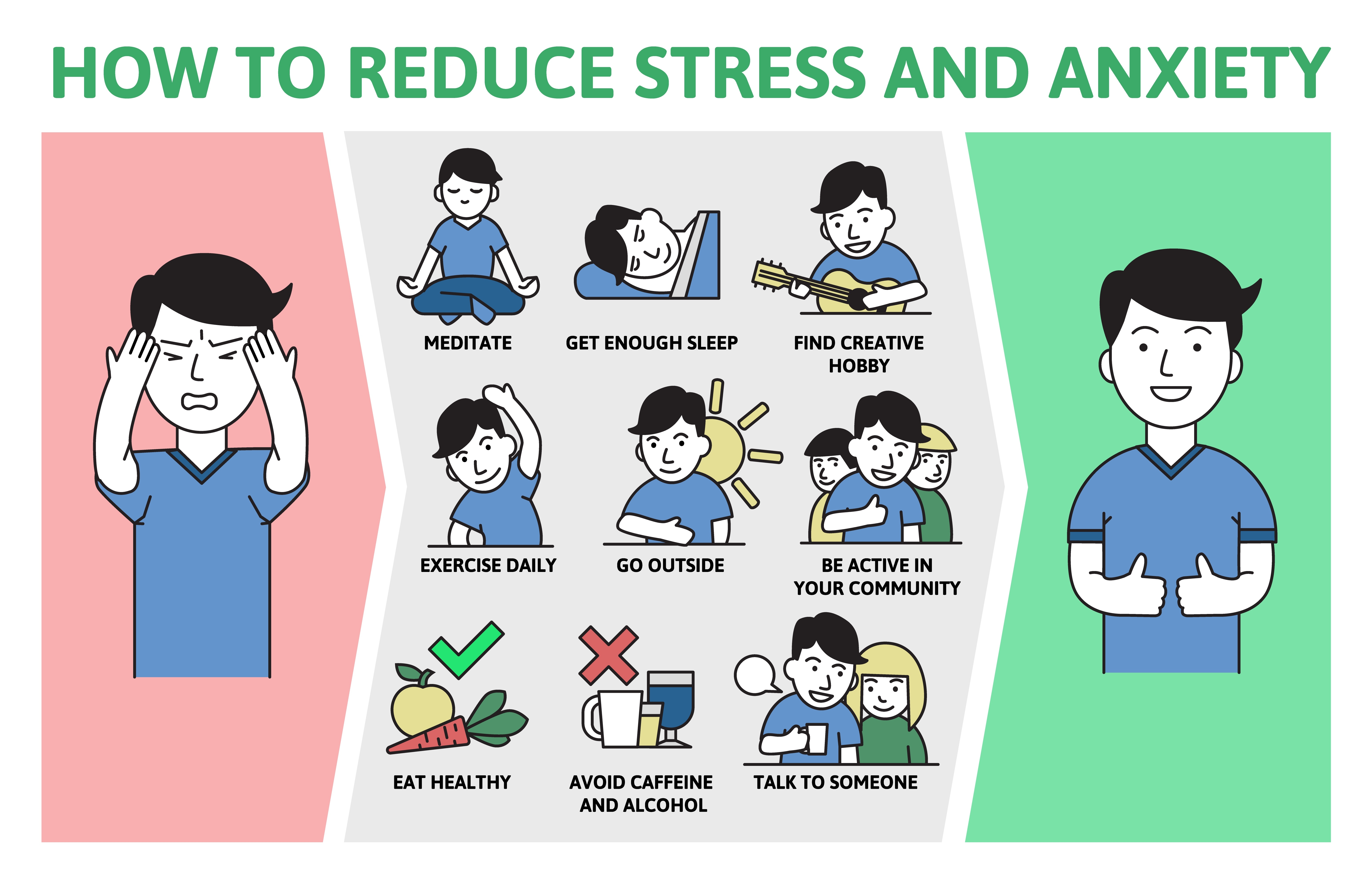
Healthy Brain Aging
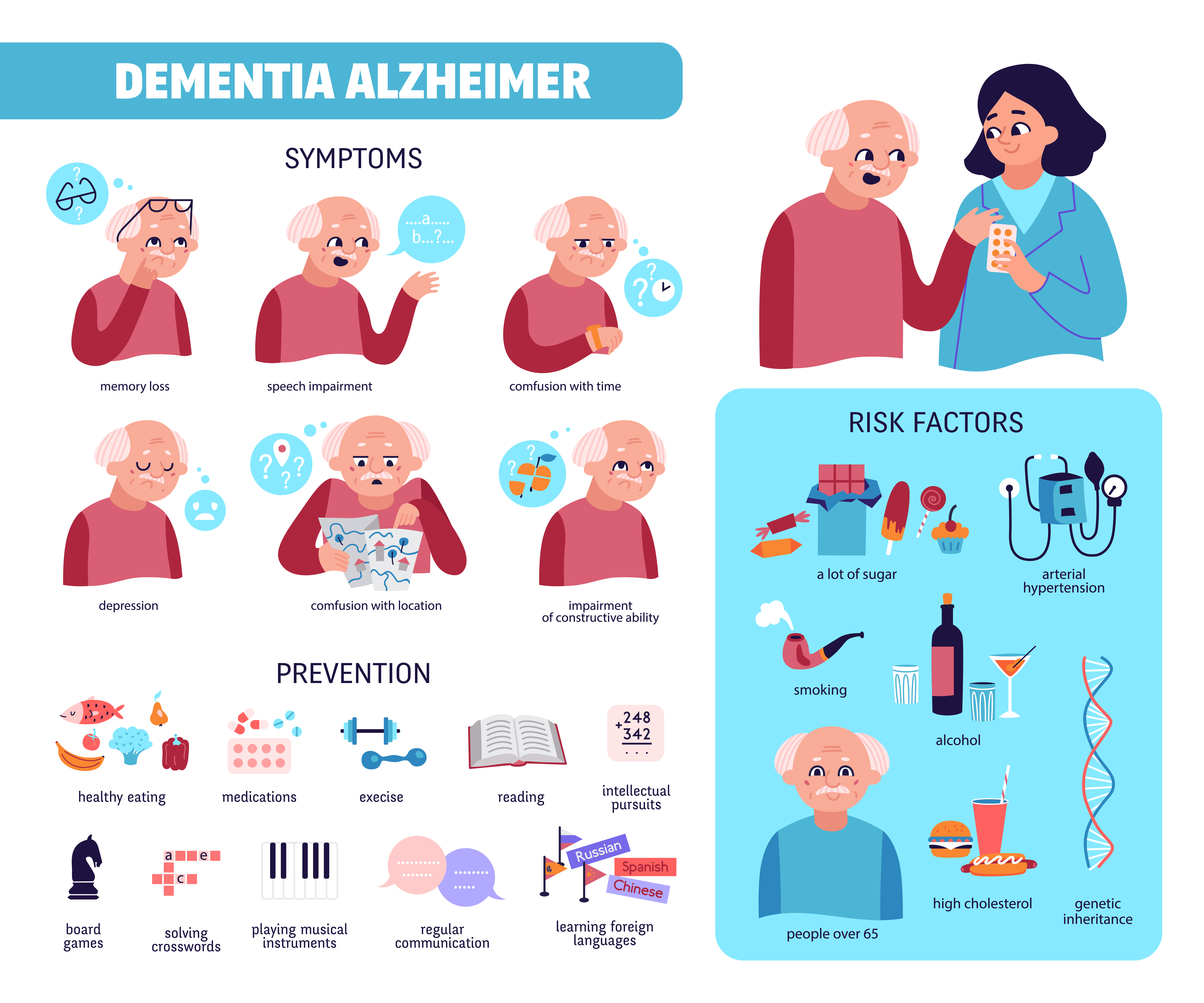
- Preventing Mild Cognitive Impairment, Dementia, Neurodegeneration, Alzheimer's disease.
- 预防轻度认知障碍、痴呆、神经退化、阿尔茨海默病。
Novel Strategies for Healthy Brain Aging
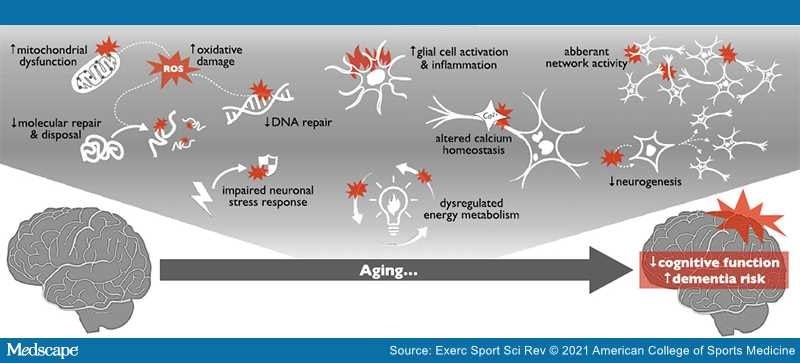
One of the best strategies for healthy brain aging is regular aerobic exercise. As the world's population ages, the incidence of dementia and neurodegenerative diseases is expected to markedly increase. One key precursor to dementia and neurodegeneration is brain aging itself. Brain aging is characterized by declines in cognitive function (primarily memory and learning, attention/processing speed, and executive function). These declines may develop into mild cognitive impairment (MCI), which increases the risk for dementia and neurodegenerative diseases like Alzheimer's disease (AD).
Dysregulated Energy Metabolism: A Central Mechanism
Dysregulated energy metabolism is an underlying contributor to all hallmarks of brain aging. During aging, fasting glucose levels increase as cells become less effective at importing glucose in response to insulin (insulin resistance). Peripheral insulin resistance and elevated fasting glucose are linked with accelerated brain aging, poorer cognitive function, and dementia.
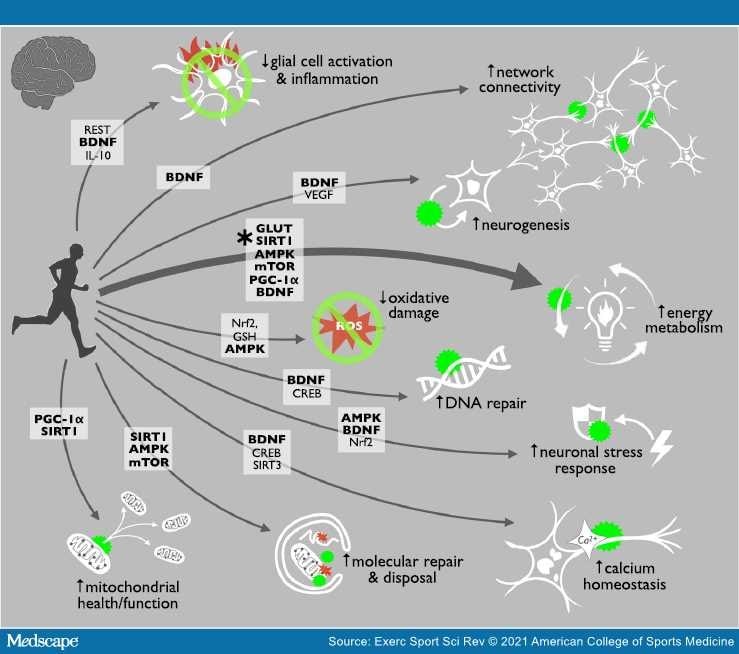
Exercise is perhaps the best strategy for inhibiting all major hallmarks of brain aging, largely because it activates key cellular energy-sensing pathways.
Exercise is an energetic stress (reduced cellular energy levels) that peripheral tissues and the brain react to with many metabolic, mitochondrial, and cellular responses (known as hormesis). These adaptive responses involve the activation of energy-sensitive pathways that restore bioenergetic homeostasis in both peripheral tissues and in the brain.
Mitochondrial Dysfunction
Aging is associated with a decline in efficiency of the electron transport chain, resulting in reduced ATP generation and electron leakage that propagates reactive oxygen species (ROS). These ROS can damage cellular structures, including mitochondria and even mitochondrial DNA (mtDNA). This mitochondrial dysfunction is a universal hallmark of aging that impairs overall tissue function, and it is exacerbated by age-related declines in mitochondrial biogenesis and disposal of damaged mitochondria (mitophagy).
Accumulation of Oxidatively Damaged Molecules
Oxidatively damaged biomolecules accumulate with aging as a result of oxidative stress, an imbalance between antioxidant defenses and ROS production.[3] These ROS can damage biomolecules like proteins, lipids, and DNA, as well as organelles — all of which can impair cellular function directly (because damaged components do not function properly) or indirectly (because damaged biomolecules may interfere with function in other cellular compartments). Neurons are especially sensitive to ROS because of their high, oxidizable lipid content and rate of oxidative metabolism.
Reduced Molecular Repair and Disposal
One main reason for both mitochondrial dysfunction and oxidative damage accumulation with aging is reduced activity of cellular repair and disposal systems.
Dysregulated Calcium Homeostasis
Cellular control of important ions and minerals becomes dysregulated in most aged tissues, but impaired calcium (Ca2+) homeostasis with aging is a particular problem in neurons. Ca2+ plays a role in numerous brain functions, including neurotransmission, neuronal excitability, synaptic plasticity, and long-term memory consolidation.
Impaired Adaptive Cellular Stress Response
All
cells activate stress resistance networks (e.g., antioxidant and
anti-inflammatory pathways) in response to stressors. In the brain, these
adaptive responses are important in settings of electrochemical, ionic, and
even psychological stress. With aging, however, most cells become markedly less
effective at mounting adaptive stress responses.
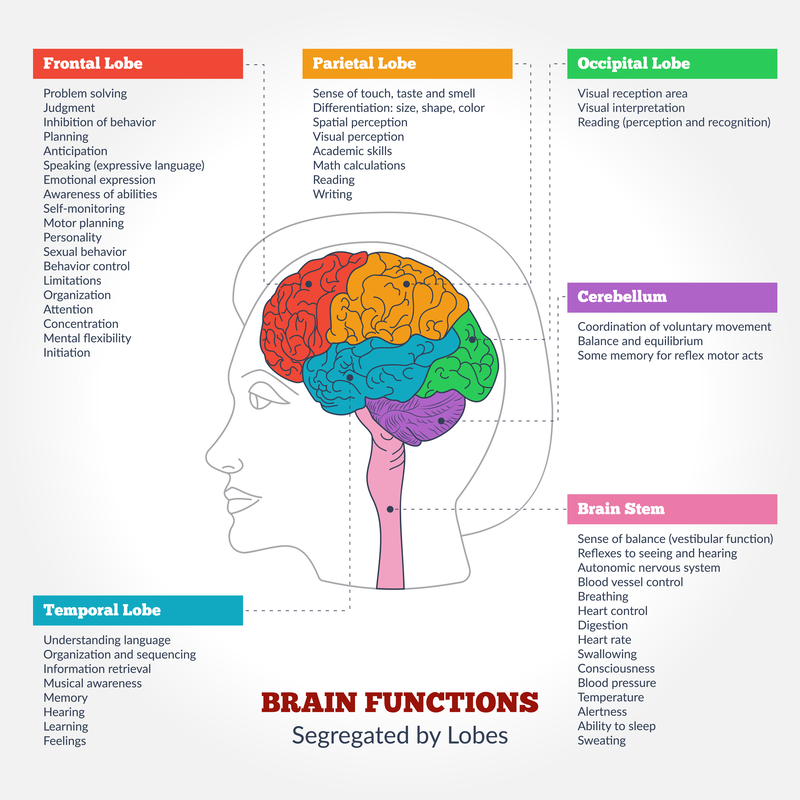
Aberrant Neuronal Network Activity
The brain relies on communication among billions of neurons. Optimal function of neuronal networks requires balanced activity of glutamatergic (excitatory) neurons and GABAergic (inhibitory) interneurons. However, during aging, activity within these circuits (largely white matter communication via myelinated axons) is dysregulated, which can result in hyperexcitability and excitotoxic damage. This may lead to degeneration of fiber systems involved in decision making and learning/memory.
Inflammation
Neuroinflammation is characterized by increased numbers of immune-activated, proinflammatory astrocytes and microglia that secrete neurotoxic cytokines.[26] This glial cell activation is linked with brain aging, MCI, and most neurodegenerative diseases. In fact, markers of neuroinflammation and related neurodegeneration [e.g., glial fibrillary acidic protein (GFAP) and neurofilament light chain (NFL)] even have been shown to increase with aging in cognitively unimpaired people.[2] Evidence shows that exercise decreases brain inflammation, marked by lower levels of the proinflammatory transcription factor nuclear factor κB and tumor necrosis factor α (TNF-α), and that these changes are associated with improved spatial memory.
Impaired DNA Repair
The accumulation of DNA damage is an important hallmark of aging, and it plays a causal role in several premature aging syndromes. DNA damage also is closely linked with brain aging, cognitive decline, and neurodegenerative diseases.[8] The genomic damage that accumulates with aging can contribute to cellular senescence (in which cells cease dividing and begin to produce proinflammatory molecules) and apoptosis (programmed cell death), leading to functional decline of organs/systems (including the brain) and reduced longevity.
Impaired Neurogenesis/Stem Cell Exhaustion
Most neurons do not proliferate, but the dentate gyrus of the hippocampus, subventricular zone of the lateral ventricle, and the olfactory bulbs all contain stem cells.[41] As is the case with most stem cells, the ability of these stem cells to self-renew and generate new progenitors (neurogenesis) declines with aging, which may account for some of the structural declines observed in the aging brain.[8] However, exercise enhances neurogenesis within these areas.
Other: Telomere Attrition and Cellular Senescence
Telomeres are repetitive DNA sequences that cap the ends of chromosomes and protect DNA from degradation. They also protect overall cellular function, as critically short telomeres can lead to cell death or senescence. Telomere shortening and cellular senescence are established hallmarks of aging in peripheral tissues. Their role in brain aging is less clear, but telomere maintenance, which contributes to the production of neuronal stem cells, may protect against neurodegenerative diseases — and there is evidence that habitual exercise is associated with longer telomeres and reduced cellular senescence in both mice and humans.
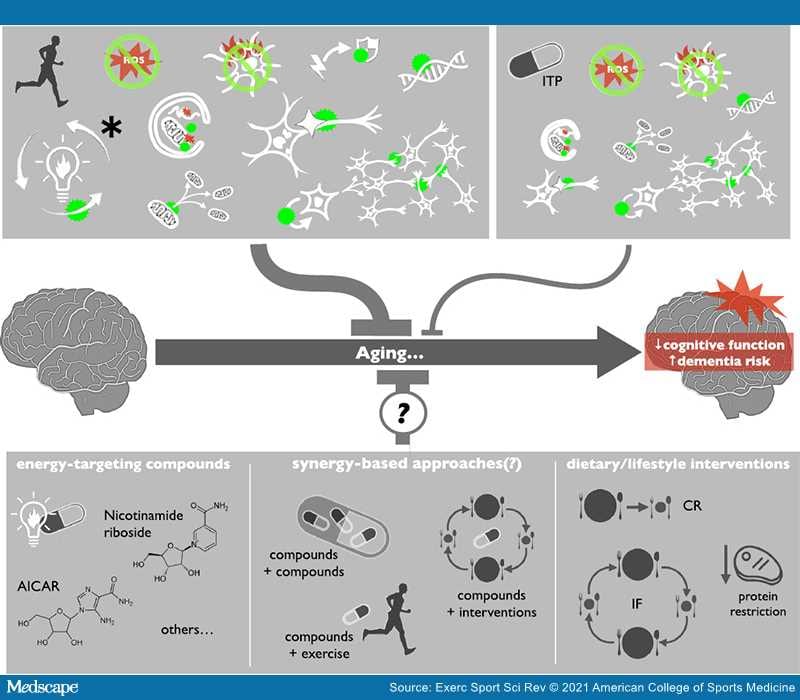
Aerobic exercise clearly has a strong, inhibitory influence on all hallmarks of brain aging.
Exercise provides a strong physiologic/metabolic stimulus and it has been suggested that exercise should be a first-line strategy for healthy brain aging. Learn more here :
Ref : https://www.medscape.com/viewarticle/948940_1
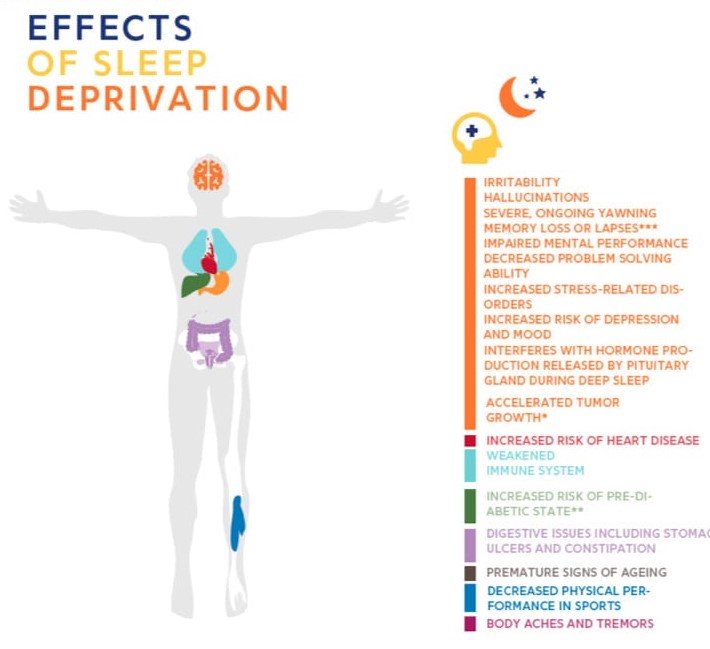
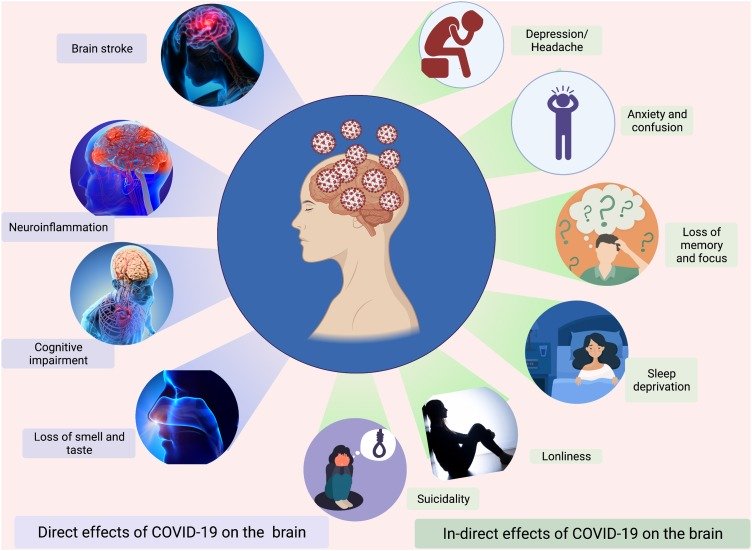
Healthy Aging 健康老龄化
The information provided in this website is for knowledge purposes only. It does not constitute medical advice.
Should you encounter any medical problem that you are unsure of, always consult your doctor or health care provider for assistance and medical advice.